A fridge full of opportunity
Commercial refrigeration is responsible for as much as 8% of total emissions attributed to airconditioning and refrigeration energy use in Australia.
It’s hard to come up with a definite figure, but commercial refrigeration may easily account for 2,000,000MWh annually.
ADVERTISEMENT
Australian Standard AS1731 sets out the minimum energy performance standards (MEPS) for commercial refrigeration, including remote and self-contained refrigerated food display cabinets.
However, there’s a lot of noncomplying equipment, and operating expenses are likely to jeopardise the thin profit margins in food retailing.
This article and others in later issues of Electrical Connection will focus on the basics and technologies of commercial refrigeration, which presents business oppportunities for electrical contractors.
The opportunities, apart from repairs and maintenance, include electrical energy consumption surveys. Think about this: the refrigerated shelves and cabinets you walk past when filling your shopping trolley may well consume 8kWh per metre per day. And such usage is usually 24/7. The political dogfight over the carbon tax has touched the refrigerant business.
The Australian Government has legislated that among the greenhouse gases designated under the Kyoto Protocol, refrigerants including hydrofluorocarbons will face an equivalent carbon price, which will be applied through existing legislation on synthetic greenhouse gas.
The seemingly unavoidable leaks of refrigerant from supermarket systems – at an average annual rate of 25% of total refrigerant installed (United States figures) – have cost the US supermarket industry dearly for decades.
That country’s Environmental Protection Agency was moved to impose regulatory limits on leak rates to curtail the environmental effect.
From a maintenance aspect, refrigerant leak testing can really pay off. The cost of a kilogram of refrigerant far outweighs that of 1,000 litres of milk.
Principles of refrigeration
Understanding refrigeration requires some knowledge of the heat processes involved, and an appreciation of mechanical aspects of such things as heat transfer, thermal load variation, insulation, etc.
In addition, process controls and energy management need to be studied. The accent here is mainly on heat processes (thermodynamics).
Refrigeration and freezing processes essentially rely on two properties of the refrigerant: its latent heat of vaporisation and latent heat of condensation.
The operational cycle of refrigerant consists of vaporisation (cooling), followed by compression then condensation (vapour to liquid) and vaporisation again.
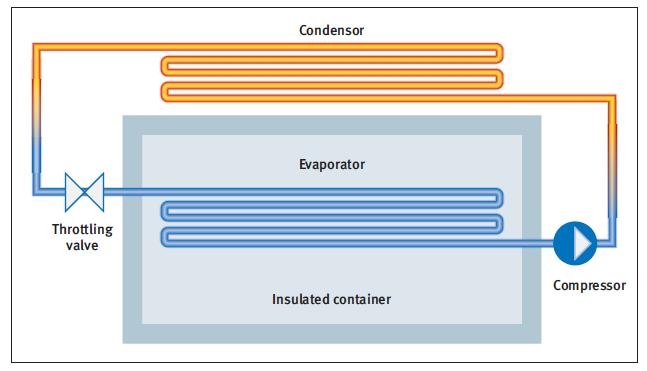
The basic mechanics involve a compressor providing suction for the evaporator, cooling the contents of a thermally insulated enclosure, and pumping the vapour back to a much higher pressure.
This is followed by condensation via heat exchange with the environment, including cooling with water, fans, etc. The subsequent vaporisation takes place by passing the refrigerant (at an elevated pressure) through a throttling valve, which provides a large pressure differential.
There is another way of cooling, using absorption and pumping followed by heating, but this will be discussed in a later article.
Latent heat
Latent heat is that which is required to achieve a change of state, for example, to go from liquid to vapour, or vice versa. During the change, the temperature remains constant.
Let’s go into a bit more depth with some thermodynamics concepts. The main terms used are latent heat and enthalpy. Figure 1 shows a simplified diagram of a refrigeration system. Cooling takes place through the evaporation process, and here is why.
Obviously cooling requires removal of heat. Heat is simply energy – no different in essence from kilowatt-hours. The measurement unit for heat is the kilocalorie, or the amount of heat needed to raise the temperature of 1kg of water by 1°C. One kilocalorie = 4184 joules (4.184kJ) = 1.16kWh.
Specific heat is the heat energy required to raise the temperature of 1kg of a substance by 1°C, thus the specific heat of water is 4.184kJ/kg.
The absorption of heat described above increases the internal energy (u) of the water, The thermally insulated box in Figure 1 contains a ‘heat load’ that needs to have its temperature reduced. This brings in the concept of specific heat again. If the insulated box is filled largely with air at atmospheric pressure it would cool by 1°C for every 1.06kJ/kg of extracted air (the specific heat of air at atmospheric pressure).
Enthalpy
We now jump to the refrigeration cycle where the refrigerant enters the evaporator as a liquid at low pressure because of compressor suction.
Evaporation requires the absorption of heat from the surroundings of the evaporator, and this is defined as the latent heat of evaporation. Part of the heat energy for vaporisation comes from what’s left in the refrigerant after the condensation phase, and the rest must be abstracted from the evaporator’s surroundings.
A typical modern refrigerant (likely a hydrofluorocarbon) has latent heat of vaporisation in the region of 220kJ/ kg. Note how much higher this value is than that of the air in the insulated box. That difference is important, because it shows the capacity the refrigerant has for extracting energy.
Now we consider enthalpy, which is a measure of the total energy of a thermodynamic system. It includes internal energy (temperature dependent) of the refrigerant and the energy needed to make room for the vapour establishing its volume and pressure.
The internal energy of the refrigerant is increased by virtue of absorbing heat – in other words, the latent heat of vaporisation. At the same time, refrigerant vapour and pressure also represent energy. (Imagine this picture: a piston being pushed by vapour pressure, as this constitutes mechanical work thus requiring more heat.)
Adding the two energies yields the enthalpy. Thus enthalpy, h, is described by the expression:
h = u + pv, where u is the internal energy, p is pressure and v is volume.
The quantities h, u, and pv are measured in kJ/kg of refrigerant. In Figure 2 the enthalpy diagram of a refrigerant is shown. The vertical axis represents pressure measured on a logarithmic scale, so as to keep the diagram on the page, and the horizontal axis indicates enthalpy.
The diagram illustrates the liquid phase (left side of the hump-shaped curve) and the vapour phase (to the right of the curve). An almost rectangular ‘box’ superimposed on the enthalpy diagram illustrates the complete refrigeration cycle of evaporation (lower horizontal trace), compression (righthand side of the box), condensation (upper horizontal line) and throttling (left-hand vertical side).
Evaporation and condensation take place at constant pressure. However, bear in mind that we are considering a cyclic process and should consider the refrigerant mass flow rate, eg: in kg/sec. Let’s use the m to indicate the mass flow rate.
The cooling phase is described by the formula m(h2 – h1) where the h values are clearly shown on the diagram and m is the mass of the refrigerant in kg/sec. The compresssion phase is described by the formula: m(h3 – h2).
The compression phase not only increases the pressure of the vapour but also its temperature. The condensation phase is described by m(h3 – h4) and takes place at a constant but high temperature. The throttling process shows no change in enthalpy (h4 = h1) but there is a big drop in pressure and temperature of the liquid refrigerant as it starts to vaporise.
The enthalpy diagram in Figure 3 is a practical one for the refrigerant R134a (tetrafl uoroethane). It looks a little confusing, but most features are explained in the caption.
A refrigeration cycle has been drawn on this diagram. The compression step m(h3 – h2), when divided into the evaporation step m(h2 – h1), provides a measure of efffectiveness. This is called the coefficient of performance (COP). Thus: COP = (h2 – h1)/(h3 – h2). Typically COP values are about 3, or more.
Refrigeration systems
How does the foregoing discussion relate to a practical installation?
Pressure and temperature measurements will determine the positioning of the refrigeration cycle on the enthalpy diagram. Measuring the pressure immediately downstream of the throttling valve (Figure 1) determines the lower horizontal line (evaporation stage). Likewise the pressure at the compressor exit provides the top trace.
Intersection of the temperature line (as determined by the temperature measurement immediately downstream of the throttling valve) with the pressure line now gives us the point h1. The point h2 is determined in the same way, as is h3. The point h4 can be inferred to vertically above h1.
Determining the power requirements of the compressor is straightforward in theory. Basically it is the cooling capacity of the evaporative phase in J/sec divided by the COP. The enthalpy diagram in Figure 3 is in kJ, so we need to multiply (h2 – h1) by 1000 to get joules (wattseconds), or to leave it in kJ so that the power is calculated in kW.
Compressor power therefore is given by P = q/COP with P in watts, with q in J/ sec, or P in kW with q in kJ/sec.
The practical aspects of refrigeration include compressors, compressor control, condensor construction and controls, evaporation and defrosting, evaporation control, and – depending on how big the refrigeration systems are – refrigerant pumps and liquid level controls.
Compressors are central, and piston types, axial fl ow and scroll compressors have to be considered. Compressor effi ciency is critical to overall effi cient operation. Control strategies such as ‘unloading’ cylinders when cooling demand is reduced, or using variable frequency drives (inverter control) to reduce compressor output, will be discussed in the next article.
Given the annual energy consumption of refrigeration systems and their importance in food production and retailing, there are business opportunities in preventive maintemance and repairs. The minimisation of refrigerant leakage, matching heat load with cooling capacity, and control strategies all ensure correct and economic operation.
-
ADVERTISEMENT
-
ADVERTISEMENT

